Delving into the realm of chemistry, let’s explore the intriguing concept of Lewis dot structure for indium. This visual representation of an atom’s electron configuration unveils insights into its chemical behavior and bonding characteristics.
As we delve deeper into the topic, we’ll unravel the significance of valence electrons, the guiding principles of the octet rule, and the fascinating world of resonance structures.
Lewis Dot Structure for Indium
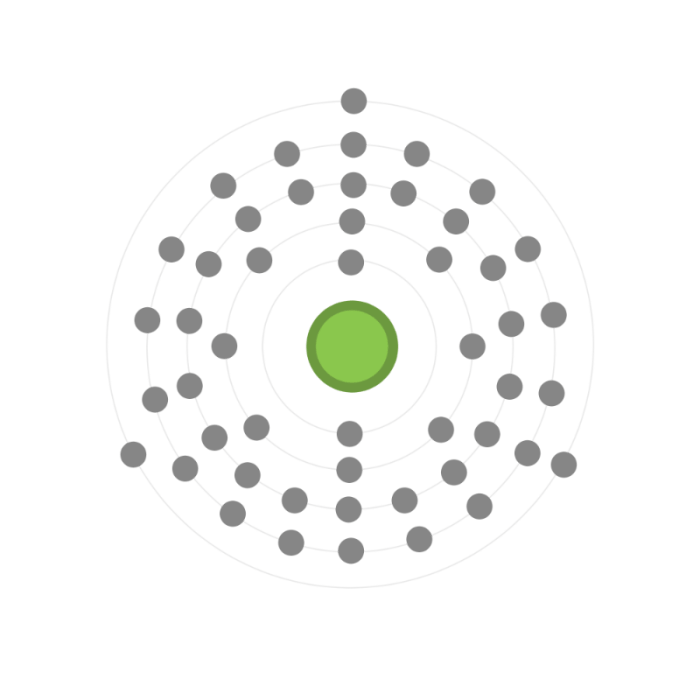
A Lewis dot structure is a diagram that represents the valence electrons of an atom or molecule. It shows the number of valence electrons and how they are arranged around the atomic nucleus. Lewis dot structures can be used to predict the chemical properties of an element or molecule.
paragraphTo draw the Lewis dot structure for indium, follow these steps:
- Find the atomic number of indium. The atomic number is the number of protons in the nucleus of an atom. Indium has an atomic number of 49, which means it has 49 protons in its nucleus.
- Determine the number of valence electrons. Valence electrons are the electrons in the outermost energy level of an atom. Indium has three valence electrons.
- Place the atomic symbol for indium in the center of the Lewis dot structure. Draw a circle around the atomic symbol to represent the nucleus.
- Place the valence electrons around the nucleus. Each valence electron is represented by a dot. The valence electrons are placed so that they are as far apart as possible.
The Lewis Dot Structure for Indium
The Lewis dot structure for indium is:“`. . .In“`
Valence Electrons and Indium
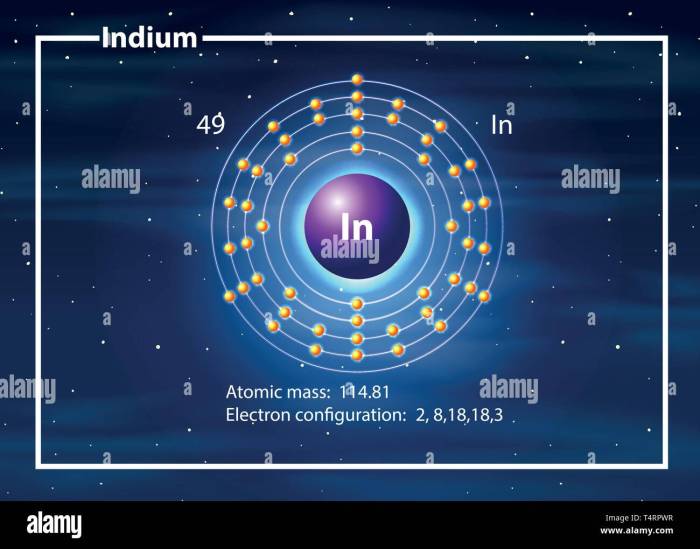
Indium, a fascinating element with atomic number 49, possesses a unique set of characteristics that make it indispensable in various technological applications. Understanding the behavior of indium in chemical reactions requires a thorough examination of its valence electrons.
Valence Electrons, Lewis dot structure for indium
Valence electrons are the outermost electrons in an atom’s electron configuration, and they play a crucial role in determining the chemical properties of an element. Indium has three valence electrons, occupying the 5s and 5p orbitals. These valence electrons are responsible for indium’s ability to form chemical bonds with other atoms, influencing its reactivity and the formation of various compounds.
Octet Rule and Indium

The octet rule states that atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons. This is because a full valence shell is more stable than an incomplete one.
Indium has three valence electrons. To achieve a stable octet, it can either gain five electrons or lose three electrons. However, it is more common for indium to lose three electrons and form a cation with a charge of +3.
The Lewis dot structure for indium is a visual representation of the element’s valence electrons. Understanding this structure is essential for predicting the chemical behavior of indium. To further enhance your understanding of chemical concepts, we recommend exploring the molar mass of lithium nitride . By examining the relationship between molar mass and chemical composition, you can gain a deeper appreciation for the intricacies of chemistry and the Lewis dot structure for indium.
Indium in Ionic Compounds
In ionic compounds, indium forms bonds with non-metals by losing three electrons and becoming a cation with a charge of +3. For example, indium chloride (InCl 3) is formed when indium loses three electrons to chlorine atoms.
Indium in Covalent Compounds
In covalent compounds, indium can share its three valence electrons with other atoms to form covalent bonds. For example, indium trichloride (InCl 3) is formed when indium shares its three valence electrons with three chlorine atoms.
Resonance Structures for Indium
Resonance structures are a way of representing the delocalization of electrons in a molecule or ion. They are used when there is more than one possible Lewis structure for a molecule or ion.
Indium can form a variety of resonance structures. For example, the indium(III) ion, In 3+, can form two resonance structures:
- In 3+with three single bonds to three chloride ions, Cl –
- In 3+with one single bond to a chloride ion and two double bonds to two other chloride ions
The two resonance structures are shown below:
The two resonance structures have the same number of valence electrons and the same overall charge. However, the electrons are distributed differently in the two structures.
Applications of Lewis Dot Structures for Indium: Lewis Dot Structure For Indium
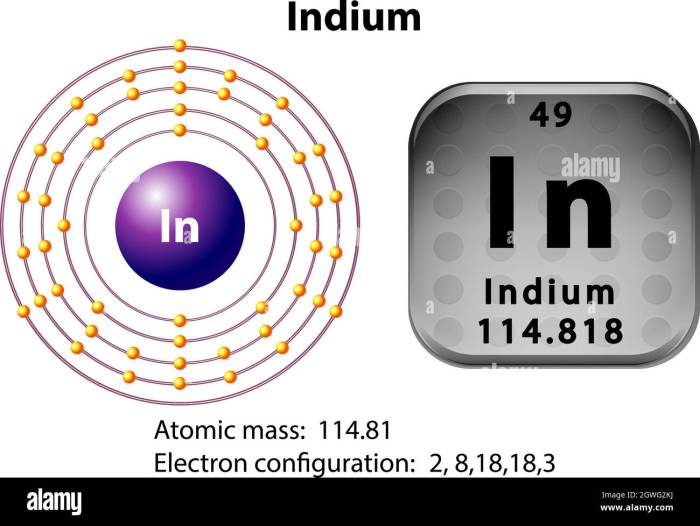
Lewis dot structures provide valuable insights into the properties and behavior of indium compounds. They help predict chemical bonding and reactivity, guiding the understanding of their structure and function.
Predicting Chemical Bonding
Lewis dot structures enable the visualization of electron pairs and their arrangement around atoms. This helps determine the type of chemical bonds formed, whether covalent, ionic, or metallic. By examining the number of valence electrons and the octet rule, chemists can predict the bonding patterns and molecular geometry of indium compounds.
Understanding Reactivity
Lewis dot structures provide clues about the reactivity of indium compounds. The presence of lone pairs of electrons or unpaired electrons indicates potential reaction sites. By identifying these reactive sites, chemists can predict the likelihood of reactions and their outcomes.
This knowledge is crucial in designing and synthesizing new materials and compounds with desired properties.
FAQ Compilation
What is the significance of valence electrons in Lewis dot structures?
Valence electrons determine the chemical reactivity of an element and play a crucial role in forming chemical bonds.
How does the octet rule apply to indium?
Indium typically follows the octet rule, which states that atoms tend to gain or lose electrons until they have a stable configuration of eight valence electrons.
What are resonance structures?
Resonance structures are alternative representations of a molecule or ion that have the same arrangement of atoms but differ in the distribution of electrons.
